CAR T-Cell Therapy
From Bench to Breakthrough: Critical Components for CAR-T Preclinical Evaluation.
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents a transformative breakthrough in cancer treatment, particularly for haematological malignancies, where it has enabled long-term remission in many patients. This innovative approach continues to shape the future of immunotherapy, opening new avenues for tackling a broader spectrum of cancers.
However, the path from concept to clinical application faces several challenges like ensuring efficient gene transfer without compromising cell viability, maintaining T cell functionality during expansion, and avoiding exhaustion or senescence.
To develop an effective CAR-T construct, it is critical to prevent off-target effects, excessive cytokine release (which can lead to cytokine release syndrome), and poor persistence in vivo. Moreover, the CAR design must avoid tonic signalling that can prematurely activate and deplete the T cells before they reach the tumour.
Rigorous preclinical testing and iterative design refinement are essential to ensure safety, specificity, durability of the therapeutic response including efficacy.
2BScientific's CAR-T Products
2BScientific Limited has a range of specialised products for key assessments which include verifying CAR expression, cytotoxicity assays to confirm tumours-killing ability, cytokine profiling, phenotypic analysis to ensure the right balance of memory and effector T cells, including T-cell profiling. All of which are essential tools at each stage of the CAR-T workflow to streamline and enhance experimental precision, as well as deepen understanding of the cellular and molecular mechanisms critical to successful CAR-T cell engineering.
We offer a comprehensive portfolio of Research Use Only reagents. Below, you will find our contributions to each stage of the CAR-T development pipeline, tailored to empower your research and accelerate progress toward clinical readiness.

Quick Links
Stage 1
Isolation of Human PBMCs from Blood
Stage 2
T- cell isolation, Purification and Expansion systems
Stage 3
T-cell expansion systems and culture monitoring systems
Stage 4
T-cell conversion to CAR construction and CAR validation assessment
Stage 5
CAR-T expansion systems ex-vivo
Stage 6
CAR-T cell monitoring, characterisation and Target Antigen screening

Stage 1: Isolation of Human PBMCs from Blood
- PBMC separation system using the Lymphoprep reagents
- Readily available PBMCs Human cells
- PBMCs Buffers for culture optimisation (HBSS, DPBS)
- Access to Humans Blood samples from patients for PBMC separation – contact KAM for further information
Stage 2: T- cell isolation, Purification and Expansion systems
Specific T-cells population are enriched with various T cell isolation procedures. Validation of these T cells with antibodies for phenotype assessment during isolation are available, including cell functionality assessment kits.
Magnetic Bead separation system for T cell isolation - Human CD3/CD28 T Cell Activation Magnetic Beads
T cell phenotype assessment with antibodies for Flow Cytometry application
Cell Functionality assessment with
Cytokine release assessment
Stage 3: T-cell expansion systems and culture monitoring systems
T cell expansion is challenging and require media components that do not affect the proliferation system and yield. Expansion of T-cell invitro requires specific activation signals to proliferate effectively before genetic modification
2BScientific Limited offers Activation ingredients for Media that can enhance the yield and efficiency. Also, at this critical stage of T cell expansion, validation on T-cell proliferation assessment, phenotyping and identifying T Cell receptor expression are essential before deciding on transformation to CAR-T.
T cell expansion Media and activation reagents
- Anti-CD3/CD28 beads or plate-bound antibodies - ActiveMax® Human T-cell Activation/Expansion CD3/CD28 Beads, premium grade from Acro
- GMP grade cytokine growth factors for media IL-2, IL-7, IL-15, IL-10, IL-21
- Human serum Albumin
- PRIME – XVT cell CDM
Other cell media components
T Cell assessment and monitoring of Cells
Stage 4: T-cell conversion to CAR construction and CAR validation assessment
Once T-cells have been successfully expanded ex vivo, the next phase focuses on engineering these cells to express a chimeric antigen receptor (CAR) which is a synthetic receptor designed to target tumour-associated antigens with precision. This stage involves a tightly controlled sequence of activation, genetic modification, and rigorous quality control validation to ensure both therapeutic potency and safety.
Expanded T-cells are prepared for genetic engineering with stimulatory agents such as Anti-CD3/CD28 beads. Following T-cell activation, CAR constructs, typically encoded within viral vectors such as lentivirus or retrovirus are introduced via transduction or transfection. This process integrates the CAR gene into the T-cell genome, enabling stable expression of the synthetic receptor on the cell surface. 2BScientific Limited offers development of CAR-T constructs and CAR-T gene delivery and validation as separate reagents or as a service provided below.
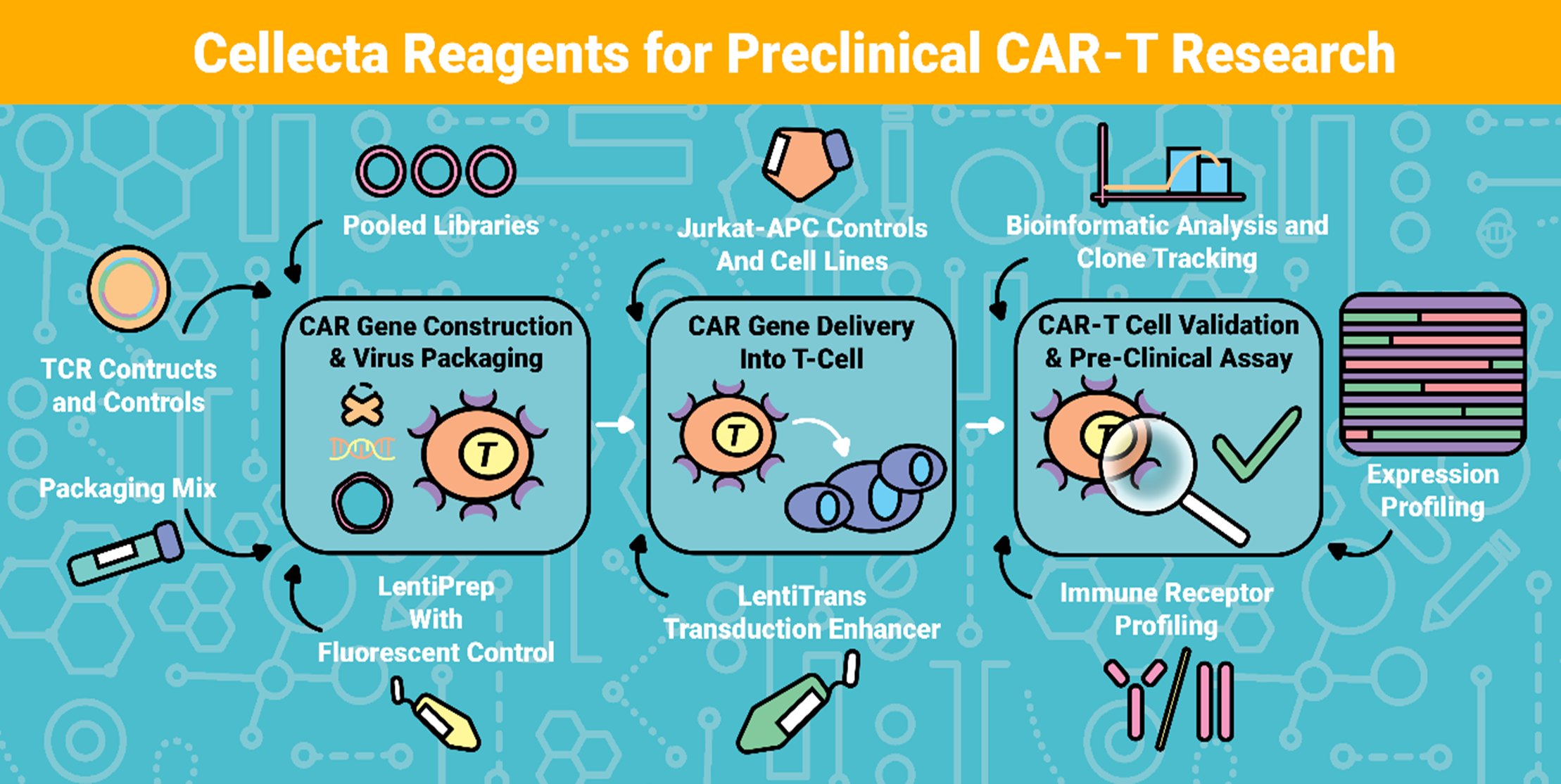
CAR Construct Optimisation reagents we can provide separately or as a service. Please note all our products and services are for RUO, so they will be used for the nonclinical or preclinical CAR-T research applications and not for CAR-T manufacturing for patient therapy.
CAR Construct Development Products for RUO | Description |
Allows production of VSV-G pseudo typed viral particles when co-transfected into packaging cells together with second or third generation HIV-based lentiviral vectors. | |
Lenti Trans Transduction Enhancer
| Boosts lentiviral transduction efficiency, especially in hard-to-transduce cells such as primary T-cells. Enhances gene delivery, leading to greater CAR expression and more consistent T-cell populations. It additionally reduces variability and improves scalability of CAR-T manufacturing. |
Lenti Fuge Viral Purification Reagent
| Used to concentrate and purify lentiviral particles after production. It ensures high-quality viral vectors for efficient transduction of T-cells. They are critical for removing impurities and increasing transduction efficiency, which directly affects CAR expression. |
T-cell Receptor Profiling to enhance CAR construct development | |
Premade and Custom Pooled TCR Libraries
| Enable high-throughput screening of T-cell receptor (TCR) variants. Useful for identifying antigen-specific TCRs or optimising CAR constructs with TCR-like specificity. Additionally assists in designing CARs with improved affinity, specificity, and reduced off-target effects. |
TCR constructs and controls
| Enable high-throughput screening of T-cell receptor (TCR) variants. Useful for identifying antigen-specific TCRs or optimising CAR constructs with TCR-like specificity. Help in designing CARs with improved affinity, specificity, and reduced off-target effects. |
Existing clinical experience with the target antigen and the expression profile of the target antigen can provide supporting information regarding potential off-tumour targets of the investigational CAR T-cells. DriverMap is a targeted RNA-seq assay that profiles expression of ~19,000 human genes. Used to monitor transcriptional changes in CAR-T cells post-engineering or post-activation. Helps to evaluate off-target effects or exhaustion signatures. | |
Jurkat NFAT-GFP Reporter Cell System - Jurkat NFAT-GFP Reporter Cell System - v1 | Jurkat T-cells engineered to express GFP under NFAT promoter control. Used to assess CAR functionality by measuring NFAT-driven GFP expression upon antigen engagement. Ideal for screening CAR constructs for activation potential and signalling fidelity. To detect GFP fluorescence in live cells (excitation ~488 nm, emission ~510 nm) but can be combined with surface markers (e.g., CD69, CD25) for deeper phenotyping. |
| Lentiviral constructs for CAR can be developed as a service Bioinformatics Analysis of CAR-T cells as a service can be provided | |
CAR Construct Validation assessment:
Flow cytometry is utilised to confirm CAR expression and surface localisation in T-cells. This step ensures that the engineered receptor is correctly configured and accessible for antigen recognition. Throughout this stage, comprehensive ex vivo assessments are carried out to detect potential issues such as tonic signalling, cellular exhaustion, or off-target effects. By monitoring cell health through cytotoxicity assays, phenotype and functional integrity, profiling provides insights into activation potential and effector function meaning CAR constructs can be accurately refined to optimise its therapeutic profile prior to clinical translation. Researchers also assess the distribution of memory and effector T-cell subsets to optimise therapeutic durability to enhance persistence and long-term anti-tumour activity.
CAR Validation Product Listings | Description for CAR-T validation |
Help confirm successful CAR integration and surface expression, which is a critical during identity and potency marker analysis. Their use in non-clinical QC ensures that the CAR construct is structurally intact and functionally capable of antigen recognition. | |
Major surface expression for CD-19 targeting CAR-T cells. | |
Enhances the detection of CAR-T expression. | |
Multiplex ELISA for Human Car-T /Cytokine detection for IL2, IL-6, IL-10, IFN-γ | Functional assay validation for cytokines levels for co-stimulatory domain activity. |
Longitudinal tracking by measuring CD137 and CD45RO/CCR7 over time to identify constructs that support central memory phenotypes. These co-stimulatory molecules often incorporated into CAR constructs as a signalling domain. Most importantly it enhances persistence of CAR-T cells. | |
Expression for Activated T-cells detection. | |
DriverMap AIR TCR/BCR Profiling
| This is a next-generation sequencing (NGS)-based assay that profiles the adaptive immune receptors, including TCR and BCR clonotypes. In CAR-T therapy, it helps:
It complements CAR detection by offering deep immune profiling, especially in post-infusion monitoring and biomarker discovery. |
If using Firefly luciferase under an NFAT promoter in a different Jurkat line to quantify screening of CAR constructs. |
Stage 5: CAR-T expansion systems ex-vivo
Ex vivo expansion of CAR-T cells is a critical step in manufacturing, but it comes with several challenges that can impact the quality, consistency, and therapeutic potential of the final product. One major difficulty is maintaining T-cell viability and functionality during prolonged culture. Overexpansion can lead to T-cell exhaustion, senescence, or skewing toward terminally differentiated effector cells, which compromises validity in vivo. Reducing these challenges have been observed using Serum-free media formulations which support robust growth even from low seeding densities, reducing variability and contamination risk. 2BScientific offers Media and GMP grade cytokine growth factors that can enhance the CAR-T expansion successfully without any limitations.
Ex Vivo Expansion Growth Factors | |
Ex Vivo CAR-T Expansion Media | |
Stage 6: CAR-T cell monitoring, characterisation and Target Antigen screening
Researchers often face challenges such as antigen escape, where tumour cells downregulate or lose the target antigen, leading to relapse. Another common issue is CAR-T cell exhaustion, marked by reduced proliferation and cytokine secretion over time. Additionally, tumour heterogeneity and the emergence of antigen-negative clones complicate long-term efficacy.
Therefore, assessment of CAR-T cells functionality, viability, cytotoxicity and Target Antigen Screening at prolonged time frame are essential otherwise the durability and success of CAR-T therapy could be significantly compromised when establishing Immunotherapy success
2BScientific Limited provides a range of Reagents that can ensure Functionality and Screening capability for final assessment of CAR-T developments. To ensure CAR-T cells persistence, functionality, and antigen expression, we have listed a range of products required for final pre-clinical assessment and validation.
T cell phenotype assessment with antibodies for Flow Cytometry application
Cell Functionality for Viability assessment and Cytotoxicity detection
- 7AAD
- PPI
- Annexin V
- Creative Biolabs: CAR-Manu™ LDH Cytotoxicity Assay Kit specifically designed for CAR-T applications using Spectrophotometer
- BrdU
- DAPI Fluorescent Reference Standard
Cytokine release assessment
- IL-15 ELISA
- IFN Type 1 ELISA
- Functional Assay Assessment - ArigoPLEX[R] Human CAR-T / CRS Cytokine Multiplex ELISA Kit (IL2, IL6, IL10, IFN gamma)
T Cell assessment and Monitoring of Cells
Assess CAR-T cell activity and phenotype
Markers like CD69 and CD25 are upregulated as part of the T cell activation cascade. These markers serve as functional readouts to confirm that the CAR-T cells are not only present but actively responding. Excessive CD69/CD25 without antigen exposure may indicate tonic signalling linked to exhaustion. Therefore, these markers are recommended.
- Memory markers: CD45RA, CCR7, CD62L.
- Exhaustion markers: PD-1, LAG-3, TIM-3, CD25, CD-69 , CD137 (4-1BB)
Target Antigen Screening
These reagents enable precise evaluation of CAR specificity and off-target binding, ensuring safer and more effective constructs.
- Most CD19-directed CAR-T therapies use the scFv region derived from FMC63 as the antigen-binding domain of the CAR
Assessment of CAR-T cytotoxic function:
These markers are used to assess after CAR-T cells are co-cultured with target tumour cells in in vitro killing assays, often alongside cytokine release (IFN-γ, TNF-α) and proliferation markers. As per pre-clinical assessment validation these markers are crucial to determine the CAR-T functionality when encountering tumour cells
- CD107a / LAMP-1 for Degranulation activity indicating that CAR-T cells are actively releasing cytotoxic molecules.
- Granzyme B to measures cytotoxic potential of CAR-T cells by quantifying its expression inside the cells.
- Perforin to assess cytotoxic payload presence confirming CAR-T cells have the machinery to kill target cells effectively
