Apolipoprotein CIII
Product Sizes
0.1 mg
16-16-120303-0.1MG
0.5 mg
16-16-120303-0.5MG
1 mg
16-16-120303-1MG
About this Product
- SKU:
- 16-16-120303
- Additional Names:
- ApoCIII|In Vitro Diagnostics, Cardiovascular Research, Diabetes
- Buffer:
- Frozen in 10 mM NH4HCO3, pH 7.4
- CE/IVD:
- RUO
- Extra Details:
- Apolipoprotein CIII (ApoCIII) is a 79-amino acid glycoprotein primarily synthesized in the liver and intestine, circulating on triglyceride-rich lipoproteins (TRLs) such as VLDL, chylomicrons, and HDL. Functionally, ApoCIII inhibits lipoprotein lipase (LPL) activity by displacing its activator ApoCII, thereby reducing triglyceride hydrolysis and delaying hepatic clearance of TRLs via LDL receptor-related protein 1 (LRP1) and syndecan-1 pathways. It also promotes VLDL secretion under lipid-rich conditions, exacerbating hypertriglyceridemia. Clinically, elevated ApoCIII levels (>15 mg/dL) correlate with hypertriglyceridemia, atherosclerosis, and coronary heart disease, while loss-of-function variants reduce cardiovascular risk by 40%. ApoCIII overexpression induces insulin resistance by impairing skeletal muscle glucose uptake and pancreatic B Beta-cell function, with elevated plasma levels predicting type 2 diabetes progression. Therapeutically, ApoCIII is targeted using antisense oligonucleotides (e.g., volanesorsen), which lower triglycerides by 70-80% in familial chylomicronemia syndrome, and RARA Alpha agonists like AM580 that suppress hepatic ApoCIII synthesis via HNF4A Alpha downregulation. Synthetic ApoCII mimetics (C-II-a) counteract ApoCIII-mediated LPL inhibition3, while dual APOC3/APOB gene silencing strategies show promise for combined dyslipidemias. Normal plasma concentrations (8-15 mg/dL) serve as biomarkers for cardiovascular risk stratification, particularly in diabetic populations. Emerging evidence positions ApoCIII inhibition as a dual therapeutic approach for metabolic and cardiovascular diseases.
- Formulation:
- Frozen in 10 mM NH4HCO3, pH 7.4
- Molecular Weight:
- 8,750 Da
- Physical State:
- Frozen
- Purity:
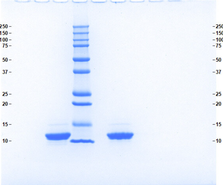
- ≥95%
- Purification:
- Liquid Chromatography Methods
- Shipping Conditions:
- Dry Ice
- Source:
- Prepared from fresh, non-frozen plasma shown to be non reactive for HBsAg, anti-HCV, anti-HBc, and negative for anti-HIV 1 & 2 by FDA approved tests.
- Storage Conditions:
- Please refer to datasheet
- Supplier:
- Athens Bioscience, Inc.
- Type:
- Proteins, Peptides, Small Molecules & Other Biomolecules: Native