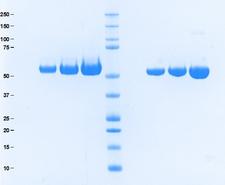
Antithrombin III
Product Sizes
0.1 mg
16-16-012020-0.1MG
1 mg
16-16-012020-1MG
10 mg
16-16-012020-10MG
About this Product
- SKU:
- 16-16-012020
- Additional Names:
- ATIII, Antithrombin, AT, Factor Xa inhibitor, Heparin cofactor|In Vitro Diagnostic, Coagulation/Fibrinolysis, Protease Inhibition, Rheumatoid Arthritis
- Application:
- Block/Inhibit/Neutralise
- Buffer:
- Lyophilized from 50 mM Tris-HCl, pH 8.0, with 150 mM NaCl.
- CAS Number:
- 90170-80-2
- CE/IVD:
- RUO
- Extra Details:
- Antithrombin III (AT III), a glycoprotein serpin superfamily, circulates in plasma at 15 mg/100 mL and exhibits higher concentrations in plasma than serum due to thrombin complex formation during coagulation. This inhibitor primarily targets proteases in the coagulation cascade, including thrombin, factor Xa, IXa, and plasmin, through a two-step mechanism: initial docking via its reactive center loop (RCL) followed by irreversible covalent complex formation. Heparin enhances AT III activity >1000-fold by inducing conformational changes in the D-helix, which accelerates protease inhibition via allosteric activation (factor Xa/IXa) or bridging mechanisms (thrombin). Clinically, congenital AT III deficiency (1:500-5000 incidence) predisposes to venous thromboembolism (VTE), with 85% of carriers experiencing deep vein thrombosis (DVT) or pulmonary embolism (PE) by age 50. Acquired deficiencies arise from hepatic dysfunction, nephrotic syndrome, disseminated intravascular coagulation (DIC), or sepsis. Reduced AT III levels (<80% activity) correlate with hypercoagulability, postoperative thrombosis, and obstetric complications like preeclampsia. Therapeutically, human plasma-derived AT III concentrates are FDA-approved for hereditary deficiency management and perioperative prophylaxis in high-risk surgeries (e.g., cardiopulmonary bypass, liver transplantation). Emerging applications include adjunct therapy for COVID-19-associated coagulopathy and modulation of inflammatory signaling via endothelial syndecan-4 receptors. AT III monitoring guides anticoagulant dosing in heparin-resistant patients, while novel siRNA therapies targeting hepatic synthesis show promise for thromboprophylaxis.
- Formulation:
- Lyophilized from 50 mM Tris-HCl, pH 8.0, with 150 mM NaCl.
- Molecular Weight:
- 58,000 Da
- Physical State:
- Lyophilized
- Purity:
- ≥95%
- Purification:
- Liquid Chromatography Methods
- Shipping Conditions:
- Blue Ice
- Source:
- Source human plasma non-reactive for HBsAG, anti-HCV, anti-HBc, and negative for anti-HIV 1 & 2 by FDA approved tests.
- Storage Conditions:
- Please refer to datasheet
- Supplier:
- Athens Bioscience, Inc.
- Type:
- Proteins, Peptides, Small Molecules & Other Biomolecules: Native