Progen
PROGEN was founded in 1983 by four scientists from Heidelberg, Germany, who joined forces to manufacture and supply high quality antibodies for biomedical research. We help scientists worldwide drive biopharmaceutical and diagnostic progress to provide high-quality and reliable treatments for patients. Our mission is to make new therapies safe and affordable and to improve existing research processes.
The PROGEN team consists of bioscientists and adeno-associated virus (AAV) experts who collaborate with specialists around the world. Our products are essentials for research in science and industry.
We are more than just a manufacturer of antibodies, AAV gene therapy tools, density gradient media, and phage display technologies: We strive to understand the needs of scientists to develop solutions to jointly address challenges in academic research, biotechnology and pharma.
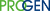
PROGEN’s antibody products are among the most published antibodies in biomedical and cell biology literature. We see one reason for this as being PROGEN’s rigorous internal validation process; indeed, the company’s antibody validation initiative by epitope mapping was highly commended by CiteAb in 2018. We frequently highlight to our customers PROGEN’s use of external independent academic quality control for its IHC antibodies, as well as pointing out the value of PROGEN’s documentation that contains comprehensive data on applications, selectivity, specificity and positive controls.
PROGEN’s antibodies are complemented by various purified and recombinant proteins to support a wide range of research requirements.
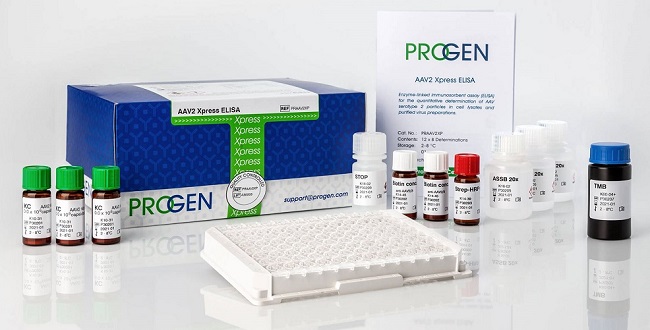
Over the past year or so, we’ve noticed a growing interest from our customers in PROGEN’s AAV titration ELISAs. These unique microtiter plate assays currently allow for the quantitation of virions and assembled empty capsids of human AAV1, AAV2, AAV3, AAV5, AAV6, AAV9 and AAV10, whereby the capture antibody detects a conformational epitope not present on unassembled capsid proteins. Each kit is supplied with an empty capsid preparation control.
Progen's new Human Antibody ELISA Kits (IgG) have arrived

Progens' Endonuclease ELISA
We are pleased to present PROGEN's most recent addition to their broad ELISA portfolio: the Endonuclease ELISA, which is intended for the in vitro measurement of endonucleases derived from Serratia marcescens, such as DENARASE® and DENARASE® High Salt, in a variety of biological samples.

Unlock the Full Potential of Your AAV Research with PROGEN’s Comprehensive Reagents
Streamline your adeno-associated virus (AAV) workflows with PROGEN's expertly designed reagents, distributed by 2BScientific. From ELISAs and antibodies to standards, Dip'n'Check tools, and OptiPrep™, PROGEN provides reliable, high-quality solutions tailored to every stage of AAV analysis and quality control. Whether you need precise quantification, rapid in-process checks, or efficient purification, PROGEN's products deliver accuracy, reproducibility, and ease of use.
Explore the PROGEN AAV Reagent Catalogue today and enhance your AAV research efficiency!
Adeno-Associated Virus Antibodies and ELISA
Adeno-Associated Virus, AAV, a nonpathogenic virus species belonging to the Parvoviridae family. AAV is classified as small (25nm) containing a single-stranded nonenveloped DNA genome. Infection with AAV only occurs with the assistance of other viruses, for example herpesvirus or adenovirus (hence the name adeno-associated virus), causing only a very mild immune response in humans. There are twelve serotypes of human AAV but the number of nonhuman AAVs exceeds 100. AAV2 is the only mammalian DNA virus that is known to integrate in a specific site of the genome.
Immunogenicity is low and the ability to infect dividing as well as non-dividing cells with stable expression make the adeno-associated virus an appealing vector for the application in gene therapy. AAV has successfully been proven as gene therapy vector with the means to attach and enter the target cell, transfer to the nucleus and express the transgene in a stable manner over a sustained period of time. In several clinical trials (e.g. FIX, CFTR, Parkinsons’s, Canavan disease) AAV did not show any serious vector-related adverse effects. Clinically relevant tissues have, however, revealed a diversified susceptibility to AAV infection. The gene transduction with AAV2 vector in muscle, retina, liver and heart resulted in lower gene expression compared to AAV serotypes 1, 5, 8 and 9 transduction in the respective tissues. The challenge of using AAV as gene therapy vector, however, lies in the resistance of some tissues to transduction with the available AAV serotypes.
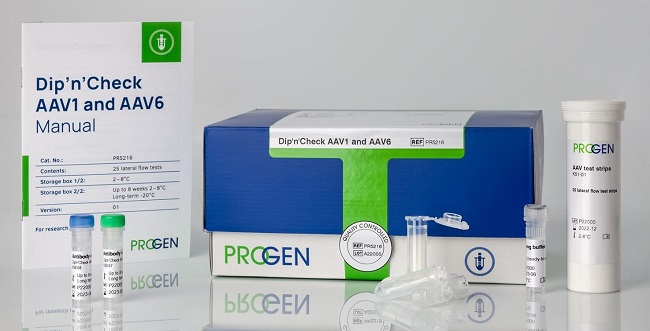 Dip’n’Check AAV1 and AAV6
Dip’n’Check AAV1 and AAV6