Detection Methods
There are many ways to detect a primary antibody on tissue. Here is a brief guide to the possibilities.
Enzymatic Detection Systems
There are many ways of detecting antibodies in immunohistochemistry, each has its own benefits and drawbacks. This is a rough guide to the possibilities – if in doubt, give us a shout!
Whichever method is used, it is important to run controls to ensure the staining is appropriate. A common negative control is to run the protocol omitting the primary antibody. This is an excellent quick way to ensure that the detection reagents are not causing unwanted staining but should not be confused with validating the primary. (Further information on this can be found in the Vector Webinars)
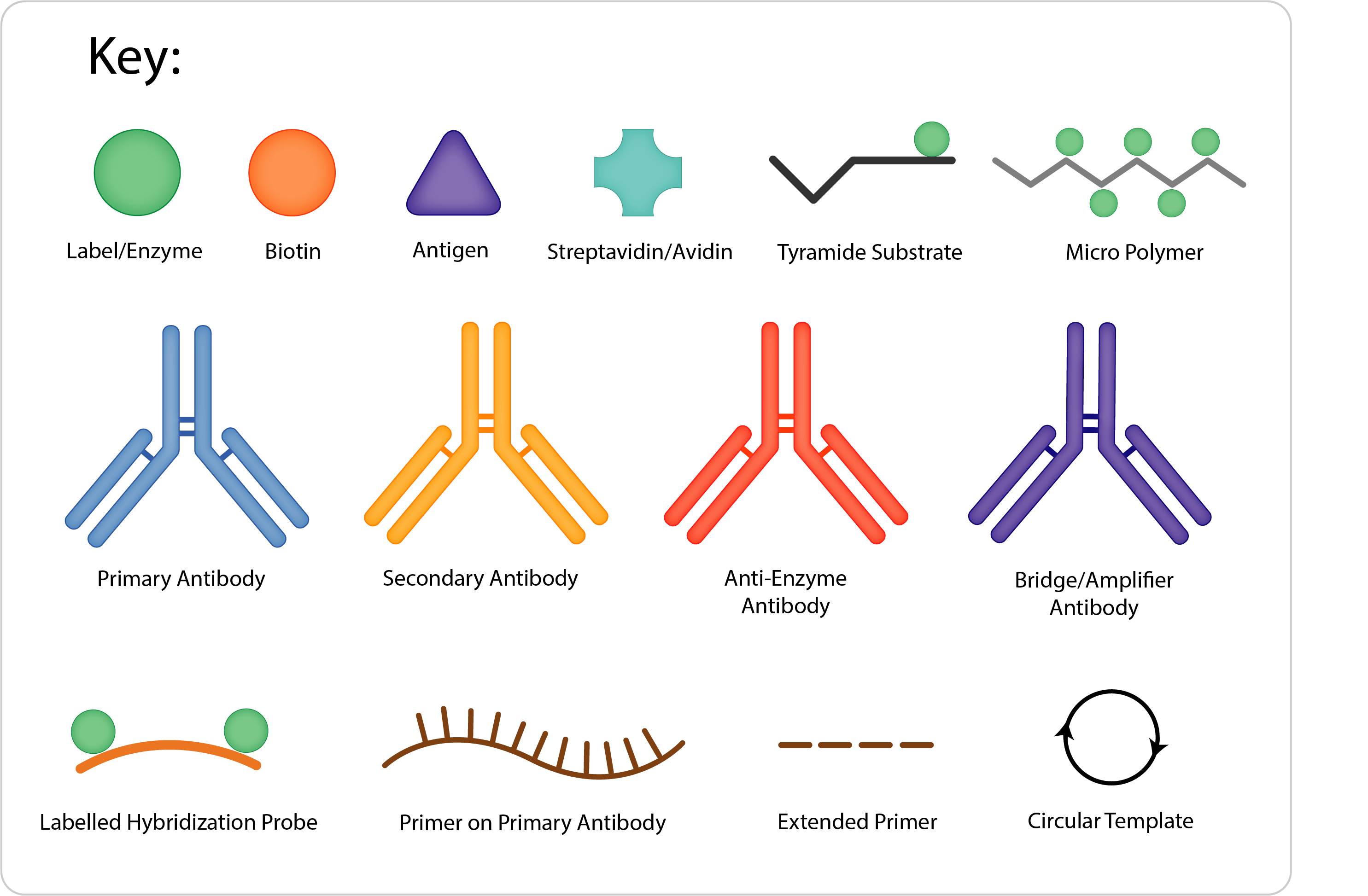
Method Diagram | Sensitivity | Method Protocol Outline | Advantages | Disadvantages |
|---|---|---|---|---|
Labelled Primary | + |
|
|
|
Indirect | ++ |
|
|
|
Peroxidase, Anti-Peroxidase (PAP) Alkaline Phosphatase, Anti-Alkaline Phosphatase (APAAP) | +++ |
|
|
|
Labelled Avidin/ Streptavidin (LAB/ LSAB) | +++ |
|
|
|
Avidin Biotin Complex ABC | ++++ |
|
|
|
Micropolymer | ++++ |
|
|
|
Amplified Micropolymer Kits | +++++ |
|
|
|
Tyramide Signal Amplification (TSA) | ++++++ |
* other variations of this method are also possible |
|
|
Rolling Circle Amplification | ++++(+++) |
|
|
|
In general, the more steps or layers there are in a protocol, the more sensitive it is. However, each layer adds time to the method, and can also increase the risk of non-specific staining.
Although some methods appear to offer unlimited amplification, the conditions within the tissue environment can limit the results in practice, and the signal to noise ratio can become problematic. A more sensitive detection system may be more expensive, but the cost can often be offset in savings on the primary antibody. If the antigen is prevalent, a very sensitive system is not required, however, if searching for the initial mutations in a tumour, more sensitivity may be very desirable. Tailoring the detection system to the experiment should therefore be considered.
For many years clinical labs have found polymer systems to be effective for most samples, and offer a slightly faster turnaround time, enabling automated staining machines to complete another run of slides each day. These polymer systems seem a practical option for many research labs, although those with more varied needs of different species of tissues and antibodies, or tighter grant can value the adaptability of the ABC systems.
The sensitivity of the method also depends on the sensitivity of the substrate used. This will be covered more in the substrate discussion, but if a little more sensitivity in a method is required, investing in a better substrate may offer a simple solution without changing the whole method.
There are further variations to these methods, some labs have used a biotin anti-(strept)avidin after an ABC layer followed by more ABC, some labs have methods that apply a substrate a second time.
Multiple Labelling
Enzymatic substrates can provide a sheltering effect over a label. This can be useful in multiple labelling experiments where the primary antibodies are made in the same species. Unfortunately, the sheltering can also interfere with the subsequent labels binding if the antigens are co-localised. Where antigens are expected in the same cell, and same cell compartment, then fluorescent methods may be required.
Vector’s IHC Multiplexing guide – for colorimetric multiple labels.
Avidin & Streptavidin – What’s the difference?
Although these 2 molecules often perform the same basic function in assays, there are some differences in these molecules that can have an impact.
Streptavidin is a non-glycosylated 60kDa protein made of 4 sub units, purified from Streptomyces avidinii. Although it has only 30% sequence similarity to avidin, it has nearly identical secondary, tertiary and quaternary structure, and a binding affinity of 1014 M-1 to biotin. However, it does have a sequence that mimics the binding sequence of fibronectin, a universal recognition domain of the extra cellular matrix that specifically promotes cell adhesion. This interaction may cause high background in some applications.
Avidin is a 68kDa basic glycoprotein purified from egg white. With an exceptionally high affinity for biotin (1015 M-1) this bond is essentially irreversible. Different methods of preparation of the avidin can result in avidin displaying modified properties; primarily deglycosylating the avidin can offer a more neutral isoelectric point, and reduces any non-specific interaction with lectins. Other treatments can affect the affinity of avidin – enabling elution of biotinylated reagents under less harsh chemical conditions.
In avidin/biotin or streptavidin/biotin blocking methods, the sections are incubated first with a solution of (strept)avidin to bind to any free biotin in tissues. The free biotin binding sites on the (strept)avidin are then filled with a solution of free biotin – which can only bind to one biotin binding site, effectively preventing further reaction.
It’s good practice to use the correct avidin or streptavidin blocking kit as appropriate, however they can be used fairly interchangeably, bearing in mind that there may be occasions where the glycosylation of avidin, or streptavidin’s mimicry of fibronectin requires the appropriate blocking kit.
It is interesting to note that anti-avidin and anti-streptavidin antibodies exist that do not recognise the other species – so where biotinylated anti-avidin is used to increase a method’s sensitivity, blocking with a streptavidin biotin blocking kit, and vice versa, may be possible.
