LEXSY – Eukaryotic Protein Expression
The Leishmania expression system LEXSY is the proprietary eukaryotic protein expression platform developed by Jena Bioscience. LEXSY is based on the protozoan host Leishmania tarentolae and was designed to combine eukaryotic protein synthesis, folding and modification with prokaryotic growth rates, simplicity and ease of handling.
Leishmania tarentolae is a robust, unicellular, flagellated eukaryotic organism circa 5 x 15 μm in size (Figure 1). It was isolated from lizards Tarentolae annularis and Tarentolae mauritanica and has been cultivated in axenic culture over decades. It is not pathogenic to mammals and is fully approved for use in biosafety level 1 laboratories (S1)
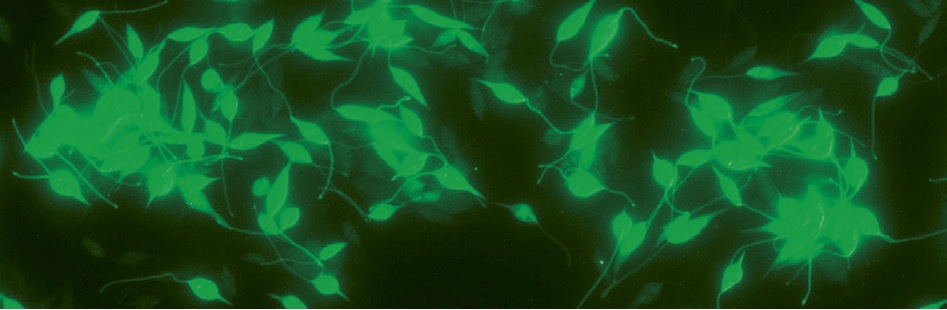
Figure 1: Leishmania tarentolae cells expressing green fluorescent protein
Overcoming limitations of other expression systems
Prokaryotic expression systems such as E. coli lack essential components for protein folding and modification and are therefore in many cases not suitable for production of functional proteins of higher organisms. Alternative eukaryotic expression systems based on e.g.mammalian or insect cells, however, have high generation times, require long development cycles and deliver low protein yields resulting in costs that are magnitudes above those of E. coli-produced proteins.
LEXSY was developed in order to overcome these limitations and combine the advantages of eukaryotic and prokaryotic expression systems: eukaryotic protein synthesis and folding/modification machinery and low generation times and ease of handling.
Features
- Correct protein folding – no inclusion bodies
- High growth rates – 6 –8 h generation time
- Full range of post-translational modifications, including: mammalian-type N-glycosylation, glypiation, phosphorylation, acetylation, prenylation, myristoylation, ADP-ribosylation, proteolytic processing and oligomerization
- High expression success rates with yields of up to 500 mg per litre of culture (Figure 2)
Protein synthesis and folding/modification machinery
Figure 2
- A: Success rates of LEXSY protein expression: Over 100 proteins have been expressed in LEXSY to date. An overall success rate of more than 90 % makes LEXSY the expression system of choice for difficult to express proteins.
- B: Yields of LEXSY expression: Over 60 % of all proteins expressed with LEXSY show yields of more than 1 mg/l of culture. Expression levels up to 500 mg/l have been achieved with some proteins.
LEXSY performs mammalian-type glycosylation
Figure 3
- C: Glycosylation in LEXSY was investigated with human erythropoietin (EPO), human interferon gamma (hu IFNγ), Toxoplasma gondiisurface antigen SAG1 and host surface glycoprotein GP63. In all cases a biantennary, galactosylated, core-α-1,6-fucosylated N-glycan structure was found that is similar to mammalian-type glycosylation (Breitling et al. 2002).
Features
- Biosafety level 1 (S1, as E. coli)
- Easy plasmid generation in E. coli shuttle vectors
- High transfection efficiencies using established electroporation protocols
- Cultivation in inexpensive media at 26 °C – no cell culture equipment necessary
- Rapid growth of LEXSY expression strains to high cell densities (109 cells/ml)
- Easy harvest and downstream processing (Figure 3).
Figure 4
- A: The LEXSY technology enables short evaluation cycles. Target genes are inserted into LEXSY expression vectors and LEXSY host is transfected by electroporation. Recombinant clones are expanded for expression evaluation in small scale suspension cultures (typically 1-10 ml). Up-scaled cultivation is used for protein production and purification. The overall procedure requires typically six weeks from cloning to purified protein.
- B: Due to fast growth of LEXSY strains in agitated suspension cultures up to 40 generations per week were achieved, whereas with insect or mammalian cells only 10 or 7 generations per week were obtained, respectively.
- C: LEXSY strains grow to cell densities known from bacterial cultures. For comparison the different host cultures were inoculated at the same density of 105 cells/ml and growth was monitored by cell counting. Following routine inoculation at 106 cells/ml, agitated laboratory LEXSY cultures reach 3x108 cells/ml within 48 h for optimal harvest of cells and protein purification (not shown). In high density fermentations up to 109 cells/ml were obtained for LEXSY cultures (Fritsche et al. 2007).
