Antibodies
This page will discuss the basics on antibodies.
Antibody Sub-Categories
Antibodies
Antibodies (Ab) are large proteins synthesised by specialised white blood called B cells, specifically B lymphocytes. They play a critical role in the body’s immune system by defending host cells from antigens, which in turn prevents more severe, faster-spreading infections. Each different protein binds to foreign entities in a lock and key fashion, where each antibody is capable of recognising an antigen epitope (small sequence) when exposed to the quaternary structure of the antibody. The quaternary structure can be defined by the antibodies’ utilisation of disulphide bonds, where a covalent bond is formed between two sulphur atoms. Sets of antibodies such as polyclonal antibodies (pAbs) may be able to recognise both the same and different epitopes of antigens, demonstrating that each antibody is not suited to bind to one specific antigen. Another key characteristic is an antibody’s ability to search and hunt for harmful foreign entities, thus preventing infection from spreading before it is detected and replicates to uncontrollable numbers [1].
Antibodies are assembled from heavy and light chains, specifically 4 peptide chains, in which two are light and two are heavy. Heavy chains consist of around 440 amino acids whereas light chains consist of 400 and are held together via a combination of disulphide (covalent) and noncovalent bonds. Each antibody has two binding sites that are symmetrically functional to bind to antigens. Once bound, it alters the chemical composition of the bound antigen, thus rendering it ineffective and unable to harm host cells. These binding sites are synthesised to defend against a wide range of antigens, whereas the rest of the antibody compromises of one of five isotypes: IgA, IgD, IgE, IgG and IgM. Each of these classes determines the function carried out once the binding site is occupied by an antigen [2].
| The Five Immunoglobulin (Ig) Classes | |||||
|---|---|---|---|---|---|
| Representation | 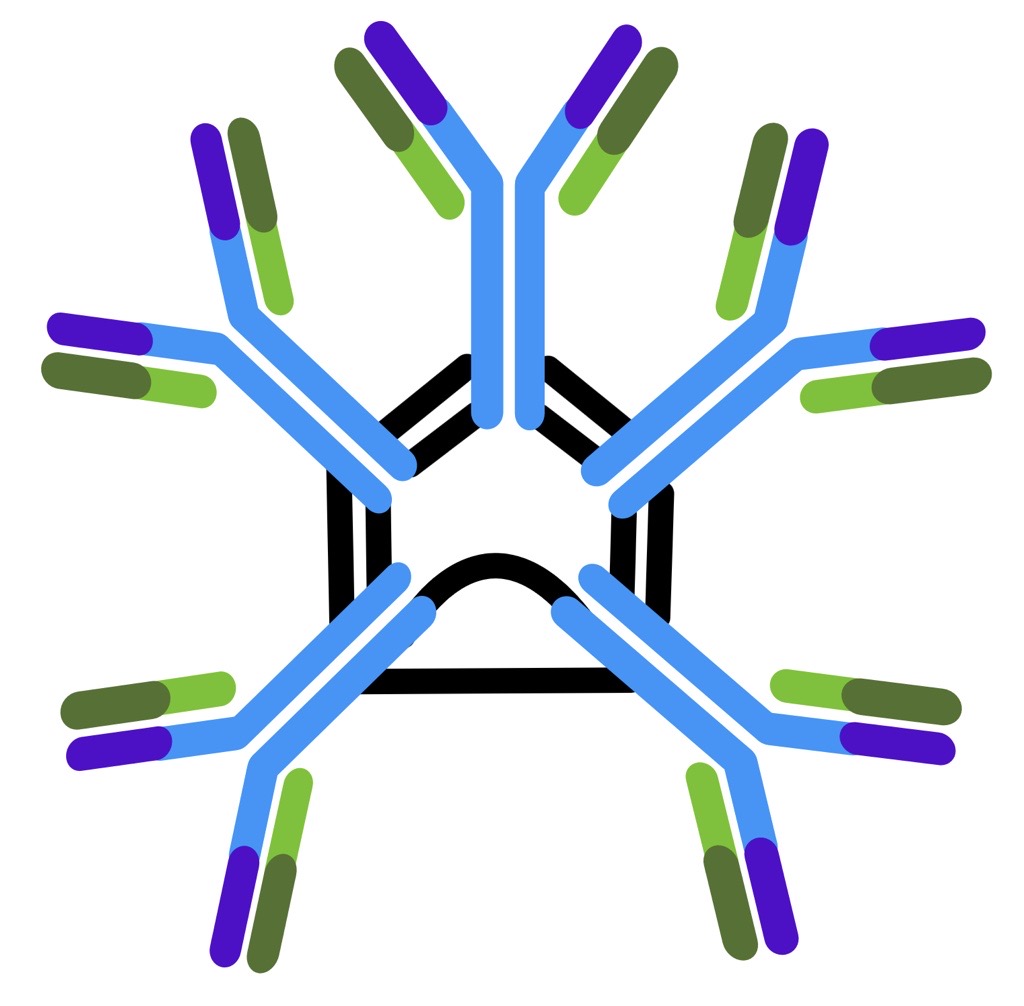 |  | 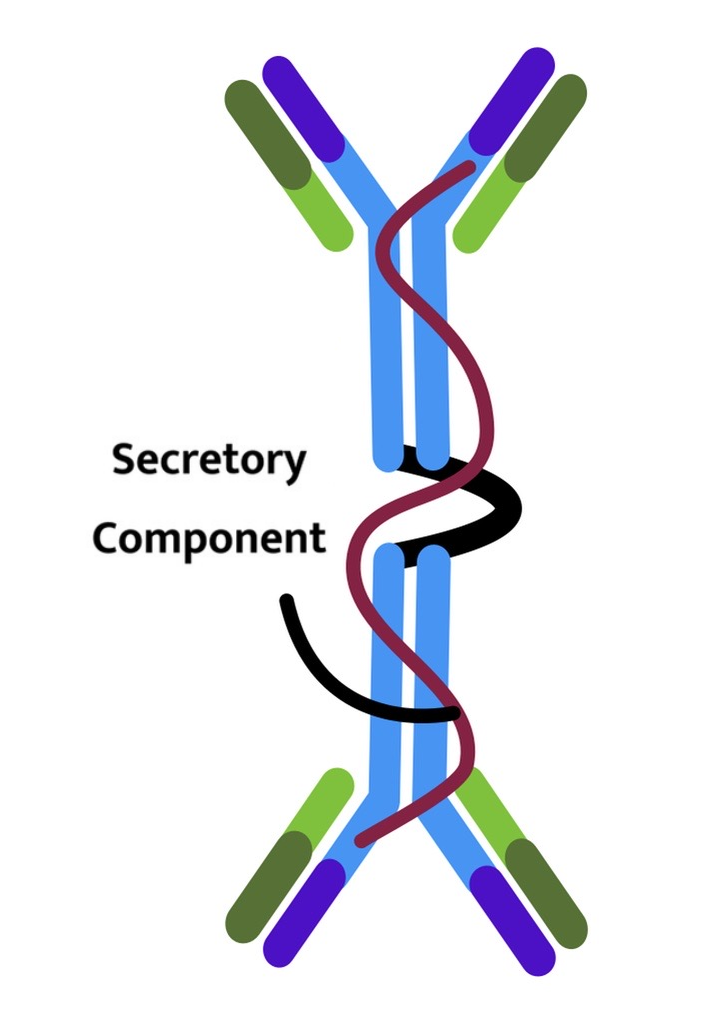.png) |  |  |
| Immunoglobulin Class | IgM | IgG | IgA | IgE | IgD |
| Number of antigen-binding sites | 10 | 2 | 4 | 2 | 2 |
| Molecular weight (Daltons) | 900,000 | 150,000 | 385,000 | 200,000 | 180,000 |
| Percentage of total antibody in serum | 6% | 80% | 13% | 0.002% | 1% |
| Crosses placenta | No | Yes | No | No | No |
| Function | The main antibody within the primary immune response, the monomer form of IgM serves as the B cell receptor. | The main blood antibody of secondary responses neutralises toxins. | Secreted into saliva, tears, mucus, and colostrum. | Antibody utilised in allergy and antiparasitic responses. | B cell receptor. |
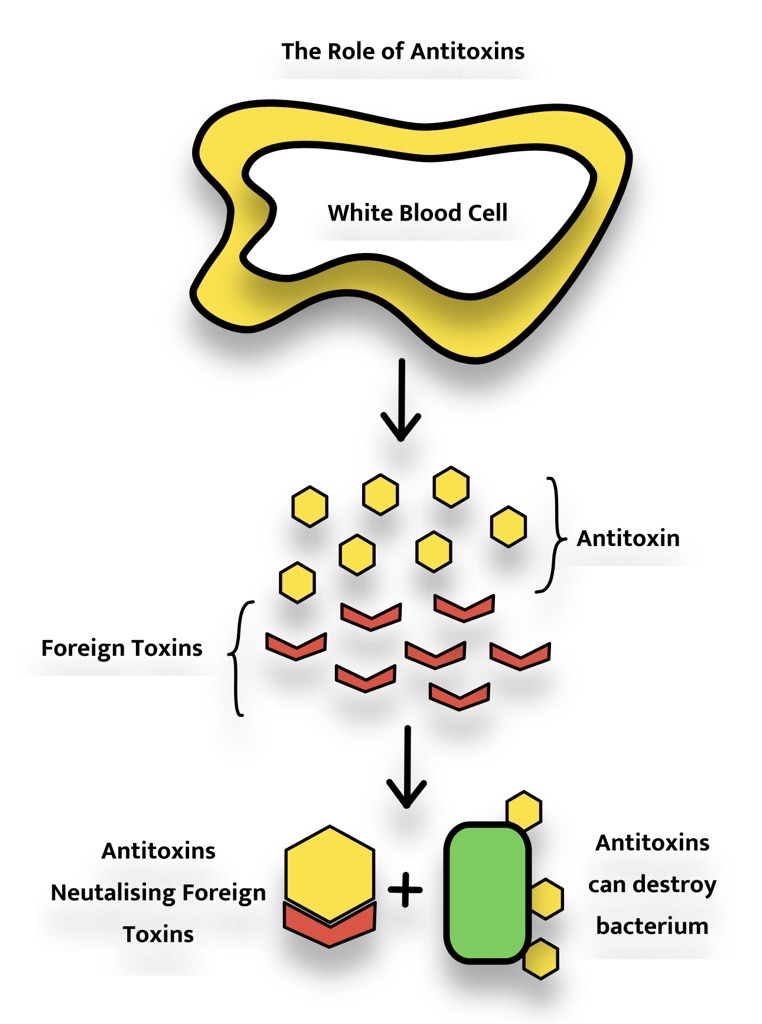
Antitoxins are a sub-group of antigens which can neutralise a toxic substance most efficiently, similarly to antibodies, each antitoxin is able to neutralise a specific toxin, they can additionally kill bacteria as well as other foreign microorganisms. Antitoxins for human application are often developed by injecting a horse with a small amount of toxin repeatedly, this results in antitoxin build-ups within blood cells which can be extracted as the antiserum. These serums can be used to treat a range of diseases including botulism, diphtheria, gas gangrene, tetanus, and dysentery [3,4].
Antibodies have a range of medical and scientific applications, this includes diagnosis, therapy, and research. Immunodiagnostic techniques such as ELISA require antibodies to detect the presence of infection-causing antigens. In addition to this, the detection of specific antibodies can demonstrate the presence of ailments such as hepatitis, in this case, an elevation in the number of IgM antibodies. With regards to therapy, monoclonal antibodies are utilised to treat a variety of diseases including multiple sclerosis, psoriasis, and breast cancer [5].
Antibodies have a huge potential in biomedical research, for example, western blotting utilises specific labelled antibodies to detect the presence of specific proteins. Immunohistochemistry and immunocytochemistry also utilise antibodies to bind to target antigens and detect the localisation via chromogenic detection with a coloured enzyme dye, or fluorescent detection with a fluorescent dye [6,7].
Finding the right antibody for the desired experiment is crucial in getting the desired results from the study or experiment. There are a range of variables that must be matched to the antibody such as the nature of the sample, this includes investigations into the desired region for detection, what processes samples will undergo before the use of antibodies and the species that synthesized the sample you wish to utilise the antibody alongside. There is a range of external websites that allow for easy selection of antibodies for the experiment, for example, Absolute Antibody allows you to find the desired primary and recombinant antibodies via the experiment or process of elimination.
2BScientific Limited has a range of products, including a selection of antibodies and antigens, be sure to utilise the filters on the left side of the site to narrow down the options. 2BScientific is passionate about ensuring that customers get the precise, required product and have a friendly and knowledgeable team ready to answer any technical queries.
