Advancing immune checkpoint inhibitor research using reagents from InVivo BioTech
Checkpoint proteins play a crucial role in regulating the immune system by preventing excessive inflammatory responses that can harm healthy cells. Cancer cells take advantage of this mechanism by overexpressing proteins in these immune checkpoint pathways, allowing them to evade detection by the immune system. To counteract this, several immune checkpoint inhibitors—antibodies that block checkpoint proteins—have been developed and approved by the U.S. Food and Drug Administration (FDA) to treat various cancers, including inhibitors of CTLA-4 and PD-1.
InVivo BioTech Services GmbH’s products to support immune checkpoint research
Providing a range of ISO9001 & ISO13485 certified reagents, InVivo BioTech Services GmbH support research into immune checkpoint signalling and cancer immunotherapy. Their range includes:
- Immune checkpoint inhibitors: including antibodies targeting PD-1, PD-L1, CTLA-4, CLEVER-1 (Stabilin-1)
- Isotype control antibodies: essential experimental controls for in vivo pre-clinical studies used in the development and validation of antibody biotherapeutics
Need for effective treatments
Cancer is a significant global health concern due to rising incidence rates1. Conventional treatments for cancer, such as surgery, radiation therapy and chemotherapy, struggle to eradicate cancer cells completely. Immunotherapy provides an alternative treatment option for advanced and difficult-to-treat cancers, especially when traditional therapies are ineffective or unsuitable. This form of treatment can address not only several types of cancer but also other diseases, such as autoimmune diseases, infectious diseases and allergy treatments.
Blocking immune checkpoints in cancer
The immune system is vital in defending the body against diseases and infections. T-cells, a key component of the immune system, are instrumental in adaptive immune responses against cancer cells. Their primary function is to identify and target cancer cells. To effectively eliminate these cancer cells, T-cells must be appropriately activated to trigger a strong immune response.
Immune checkpoints are found on the surface of T-cells and can engage with corresponding proteins in other cells. These checkpoint proteins are natural components of the immune system that regulate immune responses, serving as a safety mechanism to protect healthy cells. When these checkpoints bind to their partner proteins, they transmit an inhibitory “off” signal to the T-cells, which reduces their activity.
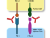
Cancer cells can hijack these immune checkpoint pathways by overexpressing inhibitory immune checkpoint proteins that can interact with cytotoxic T-cells and other immune cells to avoid destruction (Fig. 1). As a result, the cancer cells can evade detection and proliferate unchecked 2. Notable examples of checkpoint proteins include programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4).
Immune checkpoint inhibitors are a class of immunotherapy that target immune checkpoints. This approach utilises biologics, such as antibodies, to enable the immune system to identify and attack disease-causing cells by enhancing or reactivating the anti-tumour response.
PD-1/PD-L1 pathway
PD-1 is a transmembrane receptor found on the surface of immune cells, including T-cells. PD-1 is a specific receptor for the transmembrane protein, programmed death-ligand 1 (PD-L1), which is found overexpressed in certain types of cancer cells and immune cells in the tumour microenvironment3, including renal cell carcinoma (RCC), breast, colorectal, gastric and non-small cell lung cancers (NSCLC), which are associated with a poor prognosis4,5.
By overexpressing PD-L1, cancer cells are able to bind PD-1 on T-cells. This activates downstream pathways, including Src homology region 2 domain-containing protein tyrosine phosphatase 2 (SHP-2), leading to suppression of the T-cell receptor (TCR) pathway and inhibition of T-cell activity6.
Immune checkpoint inhibitors, such as PD-1 and PD-L1 antibodies, target immune checkpoints and block the binding of PD-L1 to PD-1. This mechanism allows T-cells to kill tumour cells (immune checkpoint inhibitors depicted as red antibody structures in Fig. 1).
Fig. 1. (Left) Immune checkpoint proteins
Immune checkpoint inhibitors (shown as red antibodies) are designed to promote or reactivate the anti-tumour response by blocking the interaction of immune checkpoint proteins with their partner proteins (Figure 1: immune checkpoint inhibitors, PD-1, CTLA-4).
Treatment using immune checkpoint inhibitors
Several antibody therapeutics targeting checkpoint inhibitor proteins have now been approved. The antibodies detailed below work by preventing the interaction of PD-L1 and PD-1 and enabling the enhancement of T-cell-mediated immunity.
Antibody therapeutics that target PD-1:
- Pembrolizumab (Keytruda from Merck) was the first PD-1 inhibitor approved by the FDA in 2014 for the treatment of metastatic melanoma7
- More recently, in 2024, pembrolizumab was approved by the FDA for use with pemetrexed and platinum chemotherapy as first-line treatment of unresectable advanced or metastatic malignant pleural mesothelioma (MPM)8. MPM is a highly aggressive cancer of the pleural surface (thin membrane lining the chest wall and lungs), which is predominately caused by prior exposure to asbestos9.
Antibodies that target PD-L1:
- Approved by the FDA in 2016, atezolizumab (Tecentriq®) from Genentech is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose disease progressed during or following platinum-containing chemotherapy10.
- The most recent PD-L1 antibody to be approved by the FDA is cosibelimab, marketed under the brand name Unloxcyt. In December 2024, the FDA approved the treatment for adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or radiation11.
CTLA-4 pathway
Another important class of checkpoint inhibitors is CTLA-4 inhibitors. These inhibitors enhance T-cell activity by targeting CTLA-4, an immune checkpoint protein on T-cells. CTLA-4 binds to B7 (also known as CD80) on antigen-presenting cells (APCs) and is central in regulating T-cell activation, preventing excessive immune responses.
CTLA-4 inhibitors were the first immune checkpoint inhibitors approved by the FDA, with ipilimumab (Yervoy) from Bristol-Myers Squibb receiving regulatory approval in 2011 for the treatment of melanoma. It was approved for use in both chemotherapy-naïve and previously treated patients12.
Enhancement of therapies using immune-modulating therapy
Not all patients are responsive to treatment with checkpoint inhibitors, prompting the development of complementary strategies that activate the immune system against cancer cells. One strategy whose potential is currently being explored as a monotherapy or in combination with the immune checkpoint inhibitor PD-1 is anti-Clever-1 immunotherapy. Common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1), also known as Stabilin-1, is a transmembrane receptor expressed on macrophages that interacts with various ligands.
Clever-1 plays a role in suppressing T-cell activation against tumours. Its presence has been noted on tumour-associated macrophages (TAM) in human cancers, including melanoma, lymphoma, and higher levels are linked to poorer patient outcomes13.
The effects of Clever-1 have been observed in preclinical studies using mouse models, where it supports tumour growth and metastasis formation. Research has shown that blocking Clever-1 enhances anti-tumour immunity by modifying macrophage function and promoting T-cell-mediated killing of cancer cells. This blocking can be achieved using anti-Clever-1 antibodies.
In a study published in Molecular Cancer Therapeutics, Hollmén et al. utilised the anti-Clever-1 antibody 3-372 from InVivo BioTech Services GmbH as a parental antibody to develop the anti-Clever-1 antibody, bexmarilimab (developed by Abzena). The findings indicated that bexmarilimab demonstrated promising in vivo efficacy by restoring immune responses in cancers without triggering excessive inflammation or a cytokine storm14.
Immune checkpoint inhibitors from InVivo BioTech Services GmbH
Preclinical studies of immune checkpoint inhibitors are essential for designing new checkpoint inhibitors and evaluating immune responses, drug efficacy, drug repurposing, and the long-term impact of potential toxicities. These studies provide insights into toxicity, pharmacokinetics, and biomarker identification and inform clinical translation.